Nanowerk August 18, 2023
Potentiodynamic methods that induce structural changes in Cu catalysts for the electrochemical reduction of CO2 (CO2RR) have been identified as a promising strategy for steering the catalyst selectivity towards the generation of multi-carbon products. In the current approaches, active species are created via a sequential Cu oxidation–reduction process. Researchers in Germany have shown that low-coordinated Cu surface species form spontaneously near the onset of CO2 electrocatalytic reduction. This process started by CO-induced Cu nanocluster formation in the initial stages of the reaction, led to irreversible surface restructuring that persisted over a wide potential range. On subsequent potential increase, the nanoclusters dispersed into Cu adatoms, which stabilized reaction intermediates on the surface. According to the researchers self-induced formation of undercoordinated sites on the CO2-converting Cu catalyst surface could account for its reactivity and may be exploited to (re)generate active CO2RR sites by potentiodynamic protocols…read more. Open Access TECHNICAL ARTICLE
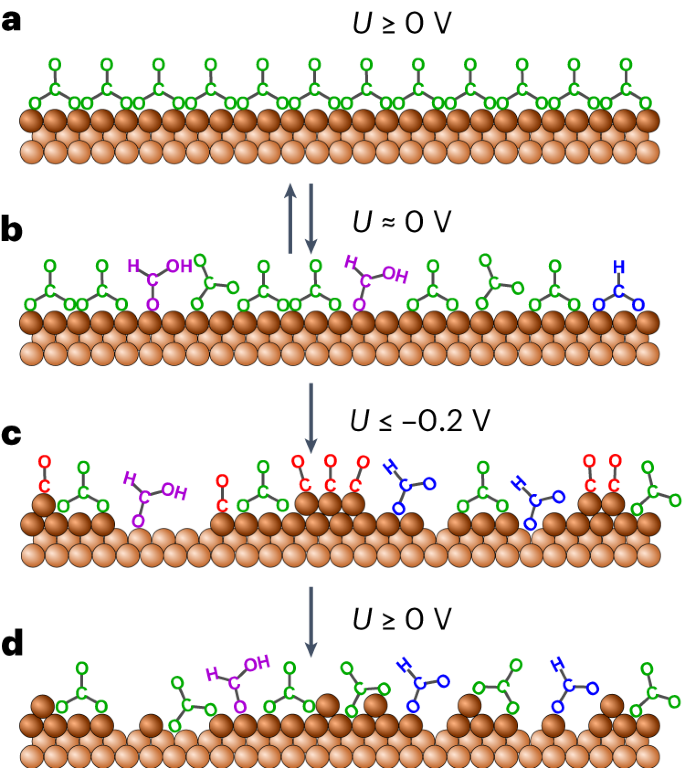
Schematic of the proposed mechanism for the formation of low-coordinated Cu species. Credit: Nature Catalysis (2023), August 17, 2023