Science Daily January 6, 2023
Lithium-sulfur batteries exhibit poor cycle life and low energy content due to the polysulfides shuttling during cycling. An international team of researchers (South Korea, USA – Argonne National Laboratory, Stanford University) developed redox-active interlayers consisting of sulfur-impregnated polar ordered mesoporous silica. Unlike the redox-inactive interlayers, these redox-active interlayers enabled the electrochemical reactivation of the soluble polysulfides, protected the lithium metal electrode from detrimental reactions via silica-polysulfide polar-polar interactions and increased the cell capacity. When tested in a non-aqueous Li-S coin cell configuration, the use of the interlayer enabled an initial discharge capacity of about 8.5 mAh cm−2 (for a total sulfur mass loading of 10 mg cm−2) and a discharge capacity retention of about 64 % after 700 cycles at 335 mA g−1 and 25 °C…read more. Open Access TECHNICAL ARTICLE
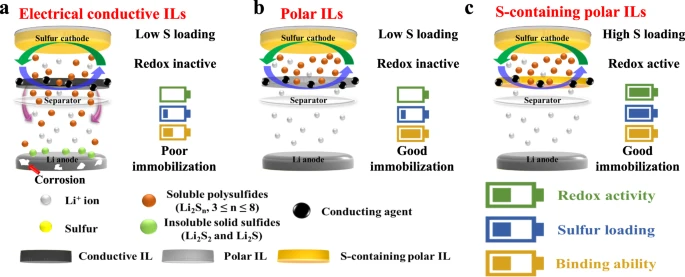
Design rationale of redox-active ILs. Credit: Nature Communications volume 13, Article number: 4629 (2022)