Nanowerk February 28, 2024
Photoinduced concerted multiple-bond rotation has been proposed in some biological systems. However, the observation of such phenomena in synthetic systems has been a challenge in the photochemistry field. Researchers in Japan described a chalcogen-substituted benzamide system that exhibits photoinduced dual bond rotation in heteroatom-containing bonds. Introduction of the chalcogen substituent into a sterically hindered benzamide system provided sufficient kinetic stability and photosensitivity to enable the photoinduced concerted rotation. The presence of two different substituents on the phenyl ring in the thioamide derivative enabled the generation of a pair of enantiomers and E/Z isomers. Using these four stereoisomers as indicators of which bonds were rotated, they monitored the photoinduced C–N/C–C concerted bond rotation in the thioamide derivative depending on external stimuli such as temperature and photoirradiation. Theoretical calculations provided insight on the mechanism of the selective photoinduced C–N/C–C concerted rotation… read more. TECHNICAL ARTICLE
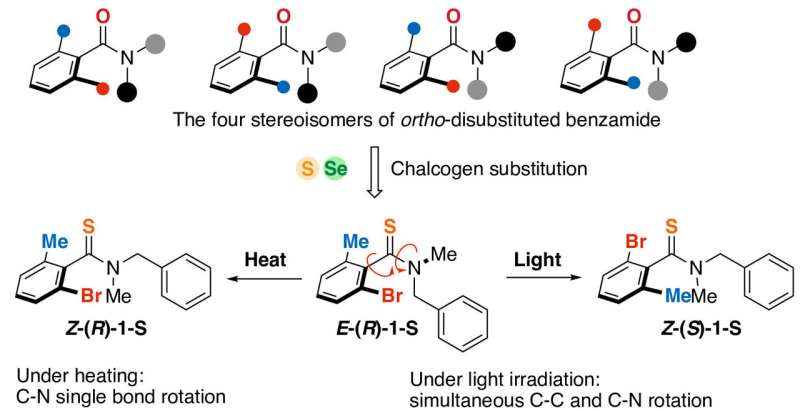
… sulfur or selenium atoms were introduced into a benzamide derivative… Credit: Nature Chemistry, 28 February, 2024