EurekAlert September 5, 2023
The versatility of the metallocenes stems from their ability to stabilize a wide range of formal electron counts. To date, d-block metallocenes with an electron count of up to 20 have been synthesized and utilized in catalysis, sensing, and other fields but, those with more than 20-electron counts have remained elusive because the metal–carbon bonds in d-block metallocenes become weaker with increasing deviation from the stable 18-electron configuration. An international team of researchers (Japan, Germany, Russia) synthesized, isolated, and characterized a 21-electron cobaltocene derivative. The discovery was based on the ligand design that allowed the coordination of an electron pair donor to a 19-electron cobaltocene derivative while maintaining the cobalt–carbon bonds. They also elucidated the origin of the stability, redox chemistry, and spin state of the 21-electron complex. According to the researchers their work reveals a synthetic method, structure, chemical bonding, and properties of the 21-electron metallocene derivative that expands the conceptual understanding of d-block metallocene chemistry. They expect their work to open previously unexplored synthetic possibilities in d-block transition metal chemistry, including the fields of catalysis and materials chemistry… read more. Open Access TECHNICAL ARTICLE
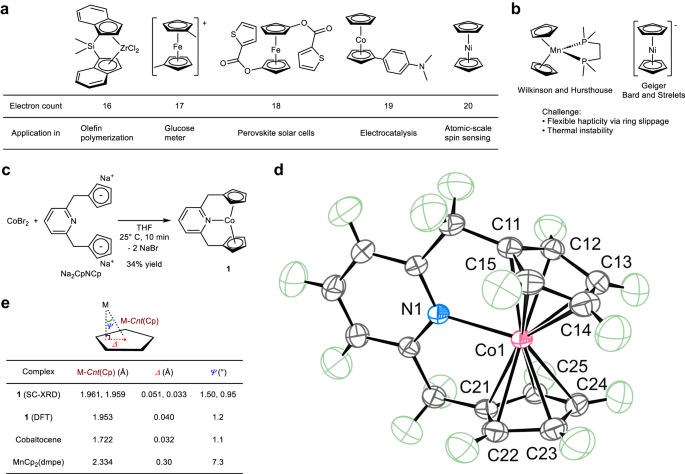
Preparation of 21-electron metallocene derivatives. Credit: Nature Communications volume 14, Article number: 4979 (2023)