Science Daily August 14, 2023
Researchers in Switzerland developed chromium compounds, very similar to those used in the past, that can replace the noble metals osmium and ruthenium. When irradiated with a red lamp the new chromium compounds the energy from the light could be stored in molecules which could serve as power source. They demonstrated it by building the chromium compounds into a stiff organic molecular framework consisting of carbon, nitrogen, and hydrogen. The stiff framework ensured that the chromium atoms were well packaged. The tailor-made environment minimized energy losses due to undesired molecular vibrations and to optimize the luminescent and catalytic properties. The disadvantage of the new materials was that chromium required a more complex framework than noble metals which further research will address. Encased chromium proved more reactive than noble metals when exposed to light. According to the researchers this paves the way for photochemical reactions that are otherwise difficult to initiate. A potential application could be in the production of active pharmaceutical ingredients… read more. Open Access TECHNICAL ARTICLE
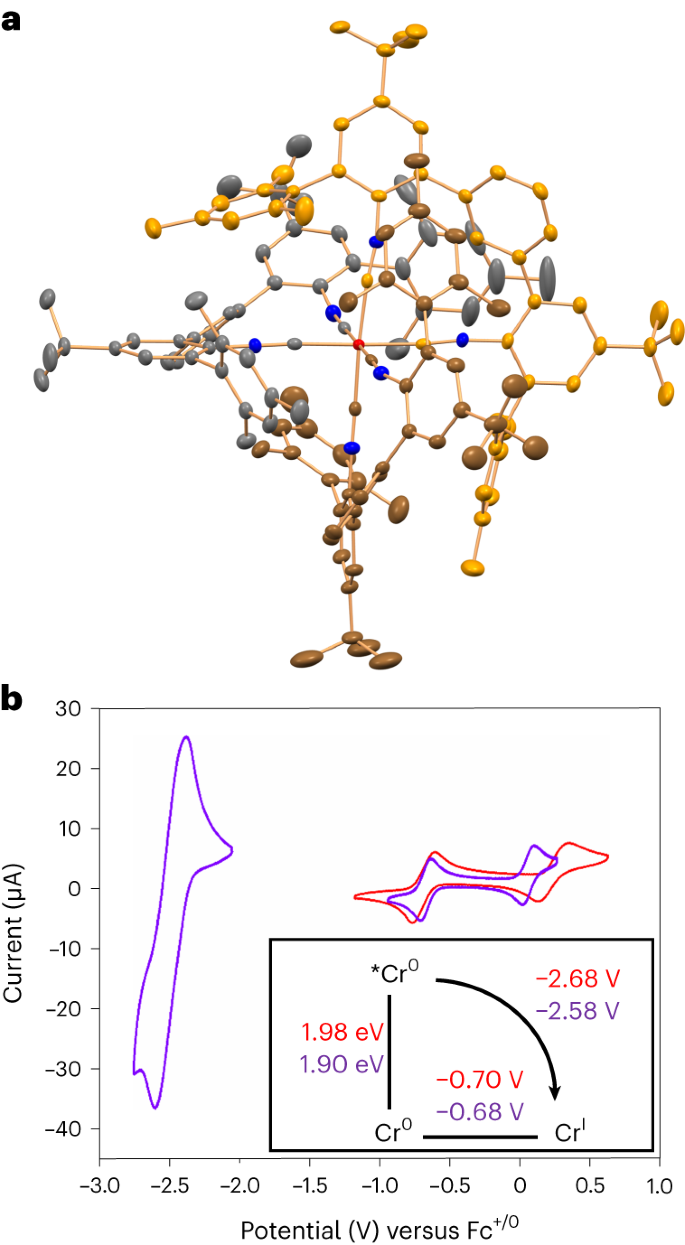
X-ray crystal structure and cyclic voltammetry. Credit: Nature Chemistry (2023), August 14, 2023