Science Daily February 22, 2023
Lithium-air batteries have scope to compete with gasoline in terms of energy density. However, in most systems, the reaction pathways either involve one- or two-electron transfer, leading to lithium peroxide (Li2O2) or lithium superoxide (LiO2), respectively. A team of researchers in the US (Illinois Institute of Technology, Argonne National Laboratory, Illinois University) used a composite polymer electrolyte based on Li10GeP2S12 nanoparticles embedded in a modified polyethylene oxide polymer matrix. They found that Li2O is the main product in a room temperature solid-state lithium-air battery. The battery was rechargeable for 1000 cycles with a low polarization gap and could operate at high rates. The four-electron reaction was enabled by a mixed ion–electron-conducting discharge product and its interface with air. The team’s battery chemistry with the solid electrolyte can potentially boost the energy density by as much as four times above batteries and it could one day power domestic airplanes and long-haul trucks… read more. TECHNICAL ARTICLE
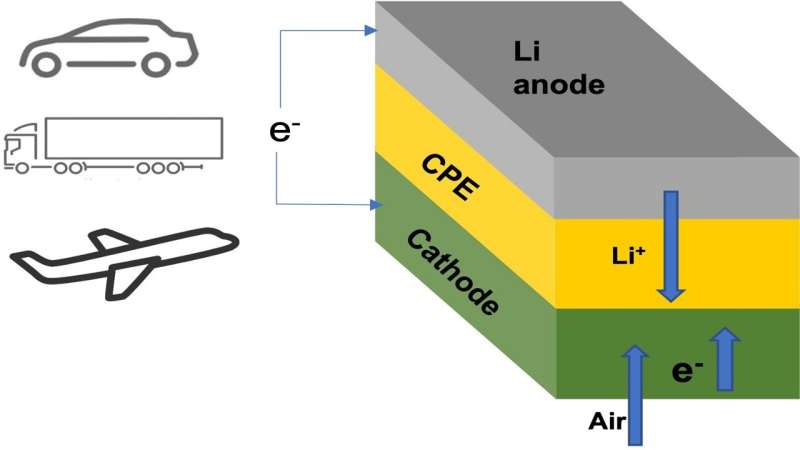
Schematic shows lithium-air battery cell consisting of lithium metal anode… Credit: Argonne National Laboratory