Phys.org February 16, 2023
Cu enables CO2-to-multicarbon product (C2+) conversion. However, the nature of the active sites under operating conditions remains elusive. A team of researchers in the US (UC Berkely, Cornell University, Lawrence Livermore National Laboratory) has presented a comprehensive investigation of the structural dynamics during the life cycle of Cu nanocatalysts. A 7 nm Cu nanoparticle ensemble evolved into metallic Cu nanograins during electrolysis before complete oxidation to single-crystal Cu2O nanocubes following post-electrolysis air exposure. They confirmed the presence of metallic Cu nanograins under CO2 reduction conditions. Other tests suggested that metallic Cu, rich in nanograin boundaries, supports undercoordinated active sites for C–C coupling. A higher fraction of metallic Cu nanograins leads to higher C2+ selectivity. A 7 nm Cu nanoparticle ensemble, with a unity fraction of active Cu nanograins, exhibited sixfold higher C2+ selectivity than the 18 nm counterpart with one-third of active Cu nanograins. The correlation of multimodal operando techniques served as a powerful platform to advance the fundamental understanding of the complex structural evolution of nanocatalysts under electrochemical conditions… read more. TECHNICAL ARTICLE
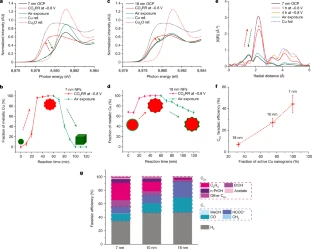
… Cu nanocatalysts during their electroreduction/reoxidation life cycle. Credit: Nature volume 614, pages262–269 (2023)