Phys.org October 27, 2022
Typically, electron transfer proceeds solely through either a metal redox chemistry or an oxygen redox chemistry without the concurrent occurrence of both metal and oxygen redox chemistries in the same electron transfer pathway. An international team of researchers (Singapore, USA – Brookhaven National Laboratory, China) has discovered an electron transfer mechanism that involves a switchable metal and oxygen redox chemistry in nickel-oxyhydroxide-based materials with light as the trigger. The proposed light-triggered coupled oxygen evolution mechanism requires the unit cell to undergo reversible geometric conversion between octahedron (NiO6) and square planar (NiO4) to achieve electronic states with alternative metal and oxygen characters throughout the oxygen evolution process. Utilizing this electron transfer pathway can bypass the potential limiting steps…read more. TECHNICAL ARTICLE
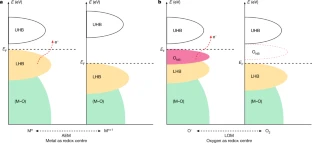
Schematic illustration of OER routes. Credit: Nature (2022)