Science Daily July 25, 2022
Alkaline Zn batteries have limited rechargeability. The short cycle life is caused by the transition between metallic Zn and ZnO, whose differences in electronic conductivity, chemical reactivity, and morphology undermine uniform electrochemical reactions and electrode structural stability. To address this an international team of researchers (Hong Kong, USA- Georgia Institute of Technology, China) has proposed an electrode design with bi-continuous metallic zinc nanoporous structures capable of stabilizing the electrochemical transition between metallic Zn and ZnO. Via in situ optical microscopy and electrochemical impedance measurements, they demonstrated the kinetics-controlled structural evolution of Zn and ZnO. In tests the storage performance of the nanoporous zinc electrodes in alkaline zinc-nickel oxide hydroxide (NiOOH) and zinc-air (using Pt/C/IrO2-based air-electrodes) coin cell configurations. The Zn | |NiOOH cell delivered an aerial capacity of 30 mAh/cm2 at 60% depth of discharging for 160 cycles, and the Zn | |Pt/C/IrO2 air cell demonstrated 80-hour stable operation in lean electrolyte condition…read more. Open Access TECHNICAL ARTICLE
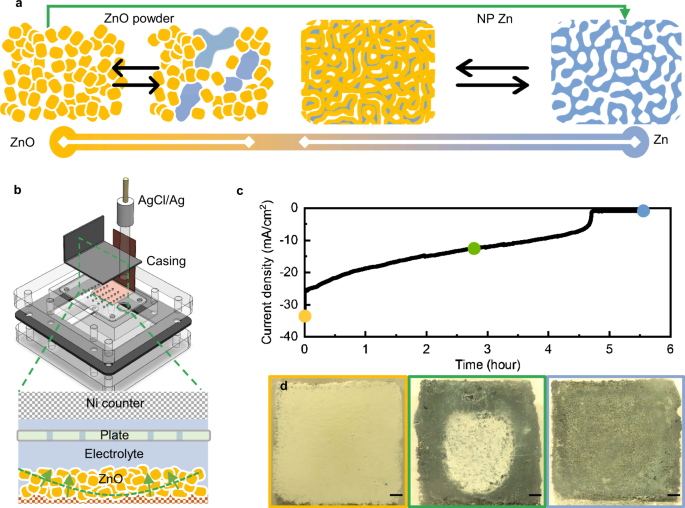
Electrochemical investigation on the transition between Zn and ZnO. Credit: Nature Communications volume 13, Article number: 2870 (2022)