Science Direct June 30, 2022
Partial oxidation of methane (CH4) to methanol (CH3OH) lifts the energy density and drives the production of numerous chemicals. In nature, this is achieved by methane monooxygenase with di-iron sites, which is extremely challenging to mimic in artificial systems due to the high dissociation energy of the C–H bond in CH4 and facile over-oxidation of CH3OH to CO and CO2. An international team of researchers (UK, China, USA – University of Chicago, Oak Ridge National Laboratory, Japan) has achieved the direct photo-oxidation of CH4 over mono-iron hydroxyl sites immobilized within a metal–organic framework, PMOF-RuFe(OH). Under ambient and flow conditions in the presence of H2O and O2, CH4 was converted to CH3OH with 100% selectivity. By using operando spectroscopic and modelling techniques, they found that confined mono-iron hydroxyl sites bind CH4 by forming an [Fe–OH···CH4] intermediate, thus lowering the barrier for C–H bond activation. The confinement of mono-iron hydroxyl sites in a porous matrix demonstrated a strategy for C–H bond activation in CH4 to drive the direct photosynthesis of CH3OH… read more. TECHNICAL ARTICLE
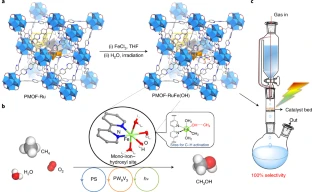
Design and synthesis of the PMOF-RuFe(OH) catalyst and the flow reactor for photo-oxidation. Credit: Nature Materials (2022)