Phys.org October 13, 2021
An international team of researchers (Australia, USA – UCLA, North Carolina State University) has developed technology to capture carbon that uses suspensions of gallium liquid metal to reduce CO2 into carbonaceous solid products and O2 at near room temperature. The nonpolar nature of the liquid gallium interface allows the solid products to instantaneously exfoliate, hence keeping active sites accessible. The solid co-contributor of silver-gallium rods ensures a cyclic sustainable process. The overall process relies on mechanical energy as the input, which drives nano dimensional triboelectrochemical reactions. When a gallium/silver fluoride mix at 7:1 mass ratio was used to create the reaction material, the efficiency was 92% at a low input energy of 230 kW∙h for the capture and conversion of a tonne of CO2. The technology is scalable and economical…read more. TECHNICAL ARTICLE
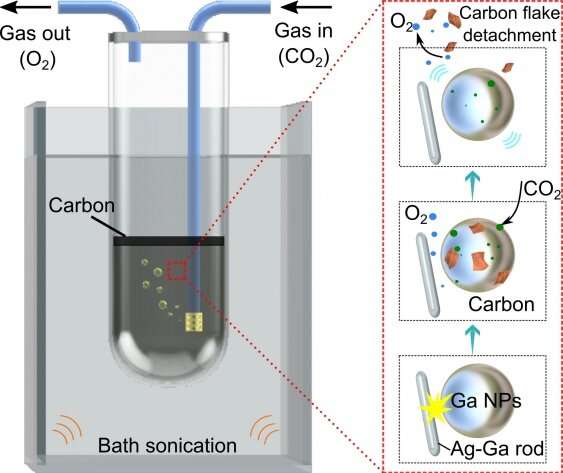
UNSW researchers have helped show how carbon dioxide can be broken down cheaply and efficiently via a process that dissolves captured CO2 gas… Credit: University of New South Wales