EurekAlert March 3, 2021
Researchers in Canada have developed a multiplexed, scalable, readily automated platform “Systematic Parallel Analysis of RNA coupled to Sequencing for Covid-19 screening” (C19-SPAR-Seq), for SARS-CoV-2 detection that can analyze tens of thousands of patient samples in a single run. To address strict requirements for control of assay parameters and output demanded by clinical diagnostics, they employed a control-based Precision-Recall and Receiver Operator Characteristics (coPR) analysis to assign run-specific quality control metrics. C19-SPAR-Seq coupled to coPR on a trial cohort of several hundred patients performed with a specificity of 100% and sensitivity of 91% on samples with low viral loads, and a sensitivity of >95% on high viral loads associated with disease onset and peak transmissibility. This study establishes the feasibility of employing C19-SPAR-Seq for the large-scale monitoring of SARS-CoV-2 and other pathogens…read more. Open Access TECHNICAL ARTICLE
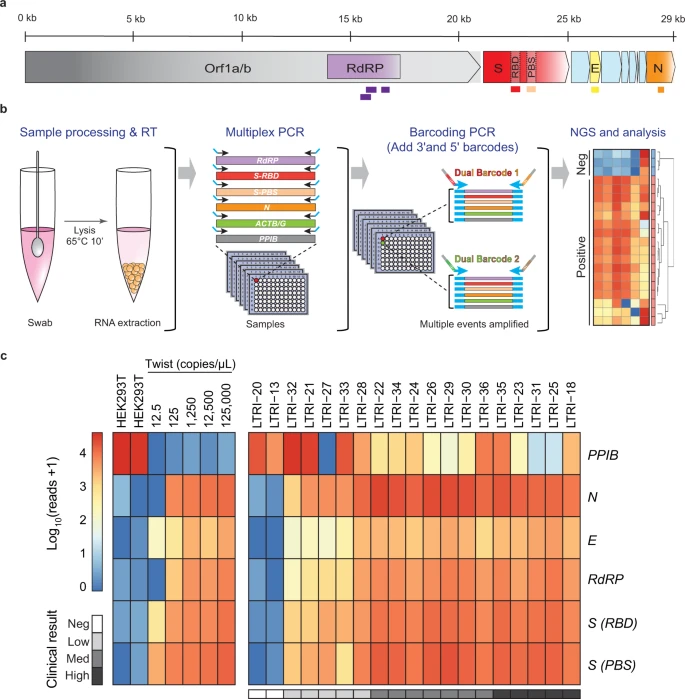
Application of C19-SPAR-Seq to detect SARS-CoV-2. Credit: Nature Communications volume 12, Article number: 1405 (2021)