Science Alert April 12, 2019
There are around 20 different molecular varieties of water– some so esoteric and rare, they may only exist inside computer simulations, or buried within distant planets. The physical confinement of water at the nanoscale can play a major role in controlling its properties. Confinement in the nanometre range can inhibit the arrangement of water molecules into an ice structure, and thereby prevent crystallisation at subzero temperature and create a state of amorphous water. Researchers in Switzerland synthesised a new class of fat molecules that form into a soft biological material called a lipidic mesophase, an extremely narrow network of connected channels, each measuring less than 1 nanometre in diameter. Cramped inside those tiny tunnels, water molecules can exist in liquid form but ice crystallisation is impossible at the molecular level – even when the lipidic mesophase was cooled with liquid helium down to around 10 Kelvin. The research provides a tool to facilitate the study of molecular structures at low temperature without ice-interfering crystals, and ultimately to understand how two main components of life, water and lipids, interact…read more. TECHNICAL ARTICLE
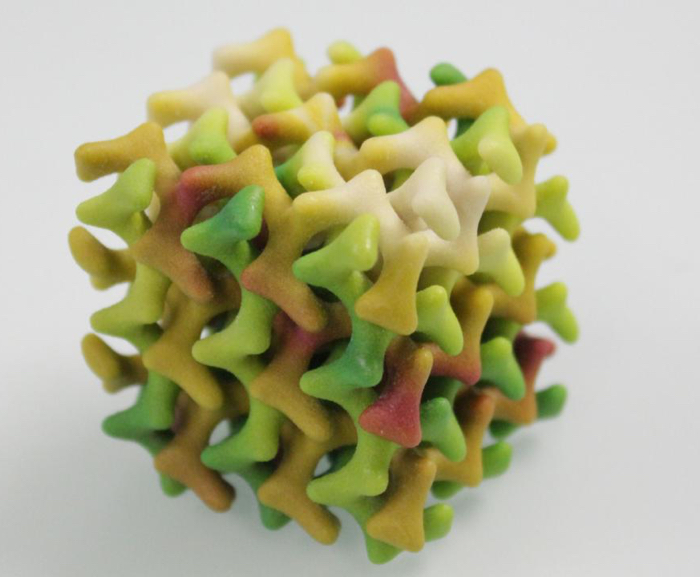
3D model of the lipid mesophase (Peter Rüegg/ETH Zurich)