Phys.org June 28, 2024
Researchers in the Netherlands identified an organic compound BHET (Bis(2-Hydroxyethyl) terephthalate) and identified and characterized an enzyme (LipA), which could degrade the PET-derived oligomer BHET. The enzyme exhibited varying sequence similarity to several BHETase/PETase enzymes. SclipA was deleted from S. coelicolor resulting in reduced BHET degradation. Overexpression of all LipA variants significantly enhanced BHET degradation. The optimum conditions were determined as pH 7 and 25 °C for all variants. The activity on BHET and amorphous PET film was investigated. S2LipA efficiently degraded BHET and caused roughening and indents on the surface of PET films, comparable to the activity of previously described TfCut2 under the same conditions. According to the researchers the abundance of the S2LipA variant in Streptomyces suggests an environmental advantage towards the degradation of more polar substrates including these polluting plastics… read more. Open Access TECHNICAL ARTICLE
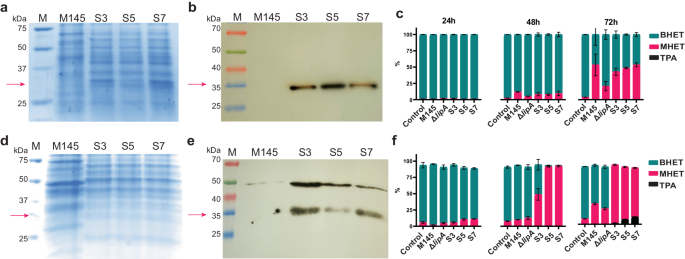
Expression of the LipA variants in S. coelicolor M145 ΔlipA in NMM and TSBS medium. Credit: Communications Biology volume 7, Article number: 725 (2024)