Phys.org June 8, 2024
Understanding how particle features influence Li and sodium (Na) co-intercalation is crucial for system design and enhancing Li selectivity. A team of researchers in the US (University of Chicago, Illinois Institute of Technology, Argonne National Laboratory, University of New York at Buffalo) investigated a series of FePO4 particles with various features and revealed the importance of harnessing kinetic and chemo-mechanical barrier difference between lithiation and sodiation to promote selectivity. The thermodynamic preference of FePO4 provided baseline of selectivity while the particle features were critical to induce different kinetic pathways and barriers, resulting in different Li to Na selectivity. They categorized the FePO4 particles into two groups based on their distinctly paired phase evolutions upon lithiation and sodiation, and generated quantitative correlation maps among Li preference, morphological features, and electrochemical properties. By selecting FePO4 particles with specific features, they demonstrated fast Li extraction from a high Li source with high purity, and high selectivity from a low Li source in a single step… read more. Open Access TECHNICAL ARTICLE
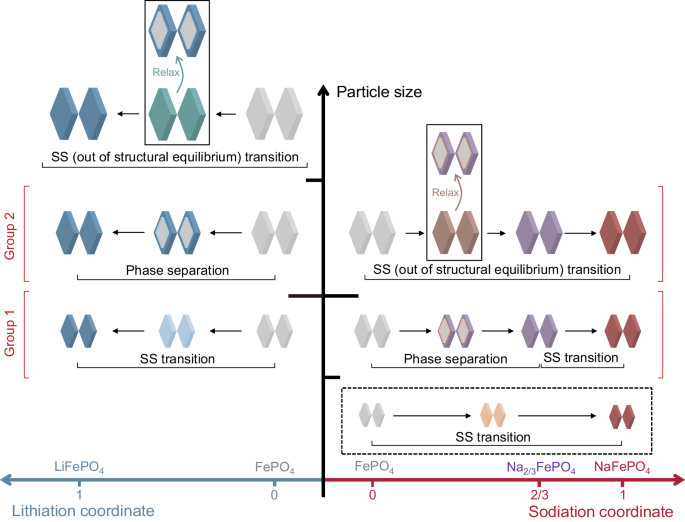
Schematic illustrations depicting the particle size dependent phase evolutions… Credit: Nature Communications volume 15, Article number: 4859 (2024)