Nanowerk November 17, 2021
A team of researchers in the US (UC Berkeley, Lawrence Berkeley National Laboratory) has improved the electrochemical reduction process for turning CO2 emissions into a fuel feedstock by developing a new approach to modify the surface of the copper catalysts used to assist the reaction. They applied a thin layer of Nafion and Sustainion, as well as a bilayer of both ionomers, to copper films supported by a polymer material, forming membranes that they could insert near one end of an electrochemical cell. For the two-layer case, they found that carbon-rich products accounted for 80% of the energy consumed by the reaction – up from 60% in the un-coated situation while feeding CO2 into the cell and applying a voltage. They concluded that the improved reaction was a consequence of the high CO2 concentration that built up in the coating layer immediately on top of copper. Additionally, negatively charged molecules that piled up in the region between the two ionomers created a low local acidity. By using a pulsed voltage with the bilayer ionomer coating, the researchers achieved a 250% increase in carbon-rich products compared to uncoated copper and a static voltage…read more. TECHNICAL ARTICLE
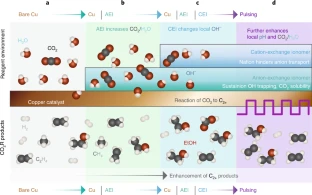
Schematic depiction of enhanced CO2R using ionomers. Credit: Nature Energy volume 6, pages1026–1034 (2021)